
Zip Express 2.7.2.1 serial key or number

Zip Express 2.7.2.1 serial key or number
|
1 AS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION ON APRIL 4, REGISTRATION NO. - - SECURITIES AND EXCHANGE COMMISSION WASHINGTON, D.C. AMENDMENT NO. 3 TO FORM S-1 REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF SANGAMO BIOSCIENCES, INC. (EXACT NAME OF REGISTRANT AS SPECIFIED IN ITS CHARTER) DELAWARE (STATE OR OTHER JURISDICTION OF (PRIMARY STANDARD INDUSTRIAL (I.R.S. EMPLOYER INCORPORATION OR ORGANIZATION) CLASSIFICATION CODE NUMBER) IDENTIFICATION NUMBER) CANAL BOULEVARD, SUITE A RICHMOND, CA () (ADDRESS, INCLUDING ZIP CODE, AND TELEPHONE NUMBER, INCLUDING AREA CODE, OF THE REGISTRANT'S PRINCIPAL EXECUTIVE OFFICES) EDWARD O. LANPHIER II PRESIDENT AND CHIEF EXECUTIVE OFFICER SANGAMO BIOSCIENCES, INC. CANAL BOULEVARD, SUITE A RICHMOND, CA () (NAME AND ADDRESS, INCLUDING ZIP CODE, AND TELEPHONE NUMBER, INCLUDING AREA CODE, OF AGENT FOR SERVICE) COPIES TO: JOHN W. LARSON, ESQ. WILLIAM J. CERNIUS, ESQ. ELIZABETH A. R. YEE, ESQ. JOSEPH G. MCCARTHY, ESQ. BROBECK, PHLEGER & HARRISON LLP LATHAM & WATKINS ONE MARKET TOWN CENTER DRIVE, 20TH FLOOR SPEAR STREET TOWER COSTA MESA, CA SAN FRANCISCO, CA () () APPROXIMATE DATE OF COMMENCEMENT OF PROPOSED SALE TO THE PUBLIC: As soon as practicable after the effective date of this Registration Statement. If the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule under the Securities Act of , check the following box. [ ] If this Form is filed to register additional securities for an offering pursuant to Rule (b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. [ ] If this Form is a post-effective amendment filed pursuant to Rule (c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. [ ] If this Form is a post-effective amendment filed pursuant to Rule (d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. [ ] If delivery of the prospectus is expected to be made pursuant to Rule , please check the following box. [ ] THE REGISTRANT HEREBY AMENDS THIS REGISTRATION STATEMENT ON SUCH DATE OR DATES AS MAY BE NECESSARY TO DELAY ITS EFFECTIVE DATE UNTIL THE REGISTRANT SHALL FILE A FURTHER AMENDMENT THAT SPECIFICALLY STATES THAT THIS REGISTRATION STATEMENT SHALL THEREAFTER BECOME EFFECTIVE IN ACCORDANCE WITH SECTION 8(a) OF THE SECURITIES ACT OF , AS AMENDED, OR UNTIL THE REGISTRATION STATEMENT SHALL BECOME EFFECTIVE ON SUCH DATE AS THE SECURITIES EXCHANGE COMMISSION, ACTING PURSUANT TO SAID SECTION 8(a), MAY DETERMINE. - -
2 THE INFORMATION IN THIS PROSPECTUS IS NOT COMPLETE AND MAY BE CHANGED. WE MAY NOT SELL THESE SECURITIES UNTIL THE REGISTRATION STATEMENT FILED WITH THE SECURITIES AND EXCHANGE COMMISSION IS EFFECTIVE. THIS PROSPECTUS IS NOT AN OFFER TO SELL THESE SECURITIES, AND IT IS NOT SOLICITING AN OFFER TO BUY THESE SECURITIES, IN ANY STATE WHERE THE OFFER OR SALE IS NOT PERMITTED. SUBJECT TO COMPLETION, DATED APRIL 4, PROSPECTUS 5,, Shares [SANGAMO LOGO] SANGAMO BIOSCIENCES, INC. Common Stock - This is our initial public offering of shares of common stock. We are offering 5,, shares. No public market currently exists for our shares. We currently anticipate the price range for the common stock to be between $ and $ per share. We intend to apply to have our common stock approved for quotation on the Nasdaq National Market under the symbol "SGMO." INVESTING IN THE SHARES INVOLVES RISK. "RISK FACTORS" BEGIN ON PAGE 5. PER SHARE TOTAL Public Offering Price $ $ Underwriting discounts $ $ Proceeds to Sangamo $ $ We have granted the underwriters a day option to purchase up to , additional shares of common stock to cover any over-allotments. NEITHER THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THESE SECURITIES OR DETERMINED IF THIS PROSPECTUS IS ACCURATE OR COMPLETE. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE. Lehman Brothers expects to deliver the shares on or about April , - LEHMAN BROTHERS CHASE H&Q ING BARINGS WILLIAM BLAIR & COMPANY ,
3 TABLE OF CONTENTS PAGE Prospectus Summary 1 Risk Factors 5 Special Note Regarding Forward- Looking Statements 17 About This Prospectus 17 Use of Proceeds 18 Dividend Policy 18 Capitalization 19 Dilution 20 Selected Financial Data 21 Management's Discussion and Analysis of Financial Condition and Results of Operations 22 PAGE Business 26 Management 43 Related Party Transactions 57 Principal Stockholders 59 Description of Capital Stock 61 Shares Eligible for Future Sale 64 Underwriting 66 Legal Matters 68 Experts 69 Where You Can Find Additional Information 69 Index to Financial Statements F-1 Until , , 25 days after the date of this prospectus, all dealers that effect transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers' obligations to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions. i
4 PROSPECTUS SUMMARY This summary highlights some of the information found in greater detail elsewhere in this prospectus. Unless otherwise indicated, information in this prospectus assumes that the underwriters do not exercise their over-allotment option, assumes the conversion of all of our preferred stock into common stock upon effectiveness of this offering and a 2-for-1 stock split which will be effected before completion of the offering. Sangamo BioSciences, Inc. is a leader in the research and development of novel transcription factors for the regulation of genes. Genes are composed of DNA and control the expression and transmission of all inherited traits. Transcription factors are proteins that turn genes on and turn genes off, or regulate gene expression, by recognizing specific DNA sequences. Our Universal Gene Recognition technology enables the engineering of a class of transcription factors known as zinc finger DNA binding proteins, or ZFPs. ZFPs are the most abundant class of transcription factors in humans and other higher organisms and naturally function to regulate gene expression. By engineering ZFPs so that they can recognize a specific gene, we have created ZFP transcription factors that can control gene expression and, consequently, cell function. We intend to establish Universal Gene Recognition as a widely used technology for commercial applications in pharmaceutical discovery, therapeutics for the treatment of human diseases, clinical diagnostics, and agricultural and industrial biotechnology. The identification of all human genes, referred to as the sequencing of the human genome, involves the dedication of enormous scientific and financial resources. The accelerating pace of genetic discovery creates significant opportunities for pharmaceutical and other life science companies. The challenge facing these companies is how to derive medically and commercially valuable knowledge from this large accumulation of new genetic information. We believe our engineered ZFP transcription factors have numerous advantages for the regulation of gene expression including: - ZFP transcription factors normally and naturally regulate gene expression in the cells of virtually all higher organisms; - ZFPs can be designed to recognize unique DNA sequences resulting in the ability to recognize a single gene within an organism's entire genome; 1
5 - ZFP transcription factors can turn on or turn off a target gene, enhancing their versatility; - ZFP transcription factors can be used to regulate gene expression in many different organisms including humans, animals, plants, fungi, bacteria and viruses; and - ZFP transcription factors can turn genes on and turn genes off in a reversible fashion, allowing regulation of gene expression for a defined period of time. To date, we have engineered hundreds of ZFP transcription factors and have performed experiments to test their ability to recognize their target sequences and to function in cells. We have also demonstrated the ability of ZFP transcription factors to regulate a limited number of commercially important genes. We intend to develop our Universal Gene Recognition technology for applications in pharmaceutical discovery, therapeutics for the treatment of human diseases, clinical diagnostics, and agricultural and industrial biotechnology. To establish Universal Gene Recognition as a widely used technology in life sciences industries, and to fund internal research and development activities, we have established and will continue to pursue collaborations with selected pharmaceutical and biotechnology companies. We have signed Universal GeneTools agreements, which we refer to as collaborations, with 18 pharmaceutical or biotechnology companies including the following companies or their subsidiaries: - Pfizer Inc., - F. Hoffmann-La Roche Ltd., - SmithKline Beecham plc, - Immunex Corporation, - Millennium Pharmaceuticals, Inc., - Pharmacia & Upjohn Company, - AstraZeneca PLC, - Genset SA, - Schering AG, - Warner-Lambert Company, - Bayer Corporation, - Merck KGaA, - Glaxo Wellcome plc, - Zaiya Incorporated and - DuPont Pharmaceuticals Company, - Procter & Gamble Pharmaceuticals. - Japan Tobacco Inc., We have also entered into a strategic partnership with Edwards LifeScience, Inc., formerly the CardioVascular Group of Baxter Healthcare Corporation, for the development and commercialization of ZFP-Therapeutics in cardiovascular and peripheral vascular diseases. Under this agreement, Baxter has purchased a $5 million convertible note which will convert into common stock upon consummation of this offering, and we have received $1 million in initial research funding from Baxter. Baxter has exercised an option by purchasing an additional $ million convertible note which will convert into common stock upon consummation of this offering for a right of first refusal to negotiate a license for additional ZFP-Therapeutics in cardiovascular and peripheral vascular diseases. We expect to enter into other strategic partnerships to accelerate the development of ZFP transcription factors as potential pharmaceutical candidates. Sangamo was founded and incorporated in Delaware in Our principal offices are located at Canal Boulevard, Suite A, Richmond, CA , and our telephone number is () 2
6 THE OFFERING Common stock offered by Sangamo 5,, shares Common stock to be outstanding after the offering 22,, shares Use of proceeds For research and development, capital equipment and general corporate purposes. See "Use of Proceeds" for more information regarding our planned use of the proceeds from this offering. Proposed Nasdaq National Market symbol SGMO The number of shares of common stock to be outstanding after this offering is based on the number of shares outstanding as of December 31, adjusted to reflect the issuance of , shares of preferred stock in January which converts into , shares of common stock upon consummation of this offering and, together with accrued interest, the issuance of a $5 million note in January and a $ million note in March which convert into common stock at the initial public offering price upon the consummation of the offering, and excludes: - a total of 1,, shares issuable upon the exercise of outstanding options at a weighted average exercise price of $ per share; - a total of , shares issuable upon the exercise of outstanding warrants at a weighted average exercise price of $ per share; and - a total of 2,, shares available for future issuance under our stock plans. 3
7 SUMMARY FINANCIAL DATA The following table sets forth summary financial data for our company. You should read this information together with the financial statements and the notes to those statements appearing elsewhere in this prospectus and the information under "Selected Financial Data" and "Management's Discussion and Analysis of Financial Condition and Results of Operations." Please see the financial statements and the notes to the statements appearing elsewhere in this prospectus for the determination of the number of shares used in computing the basic and diluted and pro forma basic and diluted net loss per share. YEAR ENDED DECEMBER 31, (IN THOUSANDS, EXCEPT PER SHARE DATA) STATEMENT OF OPERATIONS DATA: Total revenues $ 1, $ 2, $ 2, Operating expenses: Research and development 1, 4, 4, General and administrative 1, 1, Total operating expenses 2, 5, 6, Loss from operations (1,) (3,) (3,) Interest income (expense), net (55) Net loss $(1,) $(3,) $(3,) ======= ======= ======= Basic and diluted net loss per share $ () $ () $ () ======= ======= ======= Shares used in computing basic and diluted net loss per share 5, 5, 5, ======= ======= ======= Pro forma basic and diluted net loss per share (unaudited) $ () ======= Shares used in computing pro forma basic and diluted net loss per share (unaudited) 13, ======= The following table is a summary of our balance sheet as of December 31, The pro forma column reflects the issuance in January of , shares of preferred stock for $ million which converts into , shares of common stock upon consummation of this offering and a $5 million note in January and a $ million note in March which convert, together with accrued interest, into common stock at the initial public offering price upon consummation of this offering. The pro forma as adjusted column also reflects our receipt of the estimated net proceeds from the sale of the shares of common stock offered in this offering at an assumed initial public offering price of $ per share after deducting the estimated underwriting discount and offering expenses payable by us. See "Use of Proceeds" and "Capitalization" and Notes 1, 4, and 7 of Notes to Financial Statements. AS OF DECEMBER 31, PRO FORMA ACTUAL PRO FORMA AS ADJUSTED (IN THOUSANDS) BALANCE SHEET DATA: Cash, cash equivalents, and short-term investments $ 7, $21, $94, Working capital 7, 21, 94, Total assets 9, 23, 96, Long-term debt Accumulated deficit (8,) (8,) (8,) Total stockholders' equity 7, 21, 95, 4
8 RISK FACTORS An investment in our common stock is risky. You should carefully consider the following risks, as well as the other information contained in this prospectus. If any of the following risks actually occurs, it would harm our business. In that case, the trading price of our common stock could decline, and you might lose all or a part of your investment. The risks and uncertainties described below are not the only ones facing us. Additional risks and uncertainties not presently known to us or that we currently see as immaterial, may also harm our business. RISKS RELATED TO OUR BUSINESS OUR GENE REGULATION TECHNOLOGY IS UNPROVEN AND IF WE ARE UNABLE TO USE THIS TECHNOLOGY IN ALL OUR INTENDED APPLICATIONS, IT WOULD LIMIT OUR REVENUE OPPORTUNITIES. Our technology involves new and unproven approaches to gene regulation. Although we have generated some ZFP transcription factors for some gene sequences, we have not created ZFP transcription factors for all gene sequences and we may not be able to create ZFP transcription factors for all gene sequences which would limit the usefulness of our technology. In addition, while we have demonstrated the function of engineered ZFP transcription factors in cell cultures, we have not done so in animals and humans and many other organisms, and the failure to do so could restrict our ability to develop commercially viable products. If we and our Universal Gene Tools collaborators or strategic partners are unable to extend our results to new gene sequences and experimental animal models, we may be unable to use our technology in all its intended applications. Also, delivery of ZFP transcription factors into cells in these and other environments is limited by a number of technical challenges, which we may be unable to surmount. The utility of our ZFP transcription factors is in part based on the belief that the regulation of gene expression may help scientists better understand the role of human, animal, plant and other genes in drug discovery, as well as therapeutic, diagnostic, agricultural and industrial biotechnology applications. There is only a limited understanding of the role of genes in all these fields. Life sciences companies have developed or commercialized only a few products in any of these fields based on results from genomic research or the ability to regulate gene expression. We, our Universal GeneTools collaborators or our strategic partners may not be able to use our technology to identify and validate drug targets or other targets in order to develop commercial products. IF OUR TECHNOLOGY DOES PROVE TO BE EFFECTIVE, IT STILL MAY NOT LEAD TO COMMERCIALLY VIABLE PRODUCTS, WHICH WOULD REDUCE OUR REVENUE OPPORTUNITIES. Even if our Universal GeneTools collaborators or strategic partners are successful in identifying drug targets or other targets based on discoveries made using our ZFP transcription factors, they may not be able to discover or develop commercially viable products or may determine to pursue products that do not use our technology. To date, no company has developed or commercialized any therapeutic, diagnostic, agricultural or industrial biotechnology products based on our technology. The failure of our technology to provide safe, effective, useful or commercially viable approaches to the discovery and development of these products would significantly limit our business plan and future growth. 5
9 INITIAL EVALUATIONS OF OUR ENGINEERED ZFP TRANSCRIPTION FACTORS DELIVERED TO OUR UNIVERSAL GENETOOLS COLLABORATORS HAVE PRODUCED MIXED RESULTS. Some of our Universal GeneTools collaborators have been able to confirm the potential utility of our gene regulation technology. Two of our collaborators, Immunex Corporation and Millennium Pharmaceuticals, Inc., however, have not yet been able to regulate gene expression using our technology. We have taken steps to ascertain the reasons for these initial observations. We continue to work with these collaborators to address and remedy any issues that may be associated with the ZFP transcription factors, including redesign of the ZFP transcription factors. These collaborators continue to evaluate our technology. Further, most of our collaborators have not yet started testing or have not yet generated the final results of their testing. The ZFP transcription factors that we have generated for our other collaborators or our strategic partner may not function as intended and the ZFP transcription factors engineered in the future for other collaborators or strategic partners may not function as intended. If we are unsuccessful in engineering ZFP transcription factors that achieve positive results for our collaborators or strategic partners, this would significantly harm our business by reducing our revenues. IF OUR COMPETITORS DEVELOP, ACQUIRE OR MARKET TECHNOLOGIES OR PRODUCTS THAT ARE MORE EFFECTIVE THAN OURS, THIS WOULD REDUCE OR ELIMINATE OUR COMMERCIAL OPPORTUNITY. Any products that we or our collaborators or strategic partners develop using our Universal Gene Regulation technology platform will participate in highly competitive markets. Even if we are able to generate ZFP transcription factors that achieve useful results, competing technologies may prove to be more effective or less expensive which would limit or eliminate our revenue opportunities. Competing technologies may include other methods of regulating gene expression. Universal Gene Recognition has broad application in the life sciences, and competes with a broad array of new technologies and approaches being applied to genetic research by many companies. Competitive technologies include those used to map and sequence DNA, analyze the expression of genes in cells or tissues, determine gene function, discover new genes, analyze genetic information and regulate genes. Our competitors include biotechnology companies with: - competing proprietary technology; - substantially greater capital resources than ours; - larger research and development staffs and facilities than ours; - greater experience in product development and in obtaining regulatory approvals and patent protection; and - greater manufacturing and marketing capabilities than we do. These organizations also compete with us to: - attract qualified personnel; - attract parties for acquisitions, joint ventures or other collaborations; and - license the proprietary technologies of academic and research institutions that are competitive with our technology which may preclude us from pursuing similar opportunities. Accordingly, our competitors may succeed in obtaining patent protection or commercializing products before us. In addition, any products that we develop may compete with existing products or services that are well-established in the marketplace. 6
10 FAILURE TO ATTRACT, RETAIN AND MOTIVATE SKILLED PERSONNEL AND CULTIVATE KEY ACADEMIC COLLABORATIONS WILL DELAY OUR PRODUCT DEVELOPMENT PROGRAMS AND OUR RESEARCH AND DEVELOPMENT EFFORTS. We are a small company with 45 employees, and our success depends on our continued ability to attract, retain and motivate highly qualified management and scientific personnel, and our ability to develop and maintain important relationships with leading academic and other research institutions and scientists. Competition for personnel and academic and other research collaborations is intense. The success of our technology development programs depends on our ability to attract and retain highly trained personnel. If we lose the services of personnel with these types of skills, it could impede significantly the achievement of our research and development objectives. If we fail to negotiate additional acceptable collaborations with academic and other research institutions and scientists, or if our existing collaborations are unsuccessful, our technology development programs may be delayed or may not succeed. At present the scope of our needs is somewhat limited to the expertise of personnel who are able to engineer ZFP transcription factors and apply them to gene regulation. In the future, we will need to hire additional personnel and develop additional academic collaborations as we continue to expand our research and development activities and to work on some of our planned projects because these activities and projects will require additional expertise in disciplines applicable to the products we would develop with them. Further, our planned activities will require existing management to develop additional expertise. We do not know if we will be able to attract, retain or motivate the required personnel to achieve our goals. WE MAY HAVE DIFFICULTY MANAGING OUR GROWTH, WHICH MAY SLOW OUR GROWTH RATE OR GIVE RISE TO INEFFICIENCIES WHICH WOULD REDUCE OUR PROFITS. We have recently experienced, and expect to continue to experience, growth in the number of our employees and the scope of our operating and financial systems. This growth has resulted in an increase in responsibilities for both existing and new management personnel. Our ability to manage growth effectively will require us to continue to implement and improve our operational, financial and management information systems and to recruit, train, motivate and manage our employees. We may not be able to manage our growth and expansion, and the failure to do so may slow our growth rate or give rise to inefficiencies which would reduce our profits. WE ARE AT AN EARLY STAGE OF DEVELOPMENT AND MAY NOT SUCCEED OR BECOME PROFITABLE. We began operations in and are at an early stage of development. We have incurred significant losses to date, and our revenues have been limited to federal government research grants and Universal GeneTools collaborators and a strategic partner. Our Universal GeneTools collaborators are evaluating our initial ZFP transcription factors. If the initial ZFP transcription factors do not provide sufficient value to those collaborators, then they may not continue to work with us. This may also impair our ability to attract additional collaborators. As a result, our business is subject to all of the risks inherent in the development of a new technology, which includes the need to: - attract additional new Universal GeneTools collaborators and strategic partners; - attract and retain qualified scientific and technical staff and management, particularly scientific staff with expertise to further apply and develop our early stage technology; - attract and enter into research collaborations with academic and other research institutions and scientists; - obtain sufficient capital to support the expense of developing our technology platform and developing, testing and commercializing products; 7
11 - develop a market for our products; and - successfully transition from a company with a research focus to a company capable of supporting commercial activities. In addition to competitive pressures, problems frequently encountered with research, development and commercialization of new technologies and products will likely affect us. Most of our ZFP design and testing procedures take place on a relatively small scale. In the future, we intend to apply ZFP design and testing procedures at a scale involving hundreds of genes per year. We may not be able to successfully or efficiently achieve this scale. In addition, while we have had success in applying ZFP gene regulation in our laboratories, we may have difficulty in transferring our technology to our collaborators' and strategic partners' laboratories. WE ANTICIPATE CONTINUING TO INCUR OPERATING LOSSES FOR AT LEAST TWO YEARS. IF MATERIAL LOSSES CONTINUE FOR A LONGER PERIOD, WE MAY BE UNABLE TO CONTINUE OUR OPERATIONS. We have generated operating losses since we began operations in The extent of our future losses and the timing of profitability are highly uncertain, and we may not be profitable in the foreseeable future. We have been engaged in developing our Universal Gene Recognition technology since inception, which has and will continue to require significant research and development expenditures. To date, we have generated our revenues from federal government research grants, Universal GeneTools collaboration agreements and a strategic partnership agreement. As of December 31, , we had an accumulated deficit of approximately $ million. Even if we succeed in increasing our current product and research revenue or developing additional commercial products, we expect to incur losses in the near future and may continue to incur losses for at least the next two years. These losses may increase as we expand our research and development activities. If the time required to generate significant product revenues and achieve profitability is longer than we currently anticipate, we may not be able to sustain our operations. WE MAY REQUIRE FINANCING BEYOND THE PROCEEDS OF THIS OFFERING. IF WE ARE UNABLE TO OBTAIN THIS FINANCING, WE WILL BE UNABLE TO DEVELOP OUR TECHNOLOGY AND PRODUCTS. We do not know whether we will require additional financing, or that, if acquired, it will be on terms favorable to our stockholders or us. We have consumed substantial amounts of cash to date and expect capital outlays and operating expenditures to increase over the next several years as we expand our infrastructure and research and development activities. We may raise this financing through public or private financings or additional Universal GeneTools collaborations, strategic partnerships or licensing arrangements. If additional financing becomes necessary in the future, it would likely be at least tens of millions of dollars. While we believe our current financial resources and the proceeds of this offering should be adequate to sustain our operations for two years, it is not possible to estimate our financial requirements thereafter. However, to the extent we concentrate our efforts on proprietary human therapeutics, we will require FDA approval and extensive clinical trials of our potential products. This process may cost in excess of $ million per product. OUR TECHNOLOGY INFRASTRUCTURE IS NOT YET COMPLETE AND ANY DELAY OR FAILURE TO COMPLETE IT COULD PREVENT US FROM EFFICIENTLY DELIVERING ZFP TRANSCRIPTION FACTORS TO OUR UNIVERSAL GENETOOLS COLLABORATORS OR STRATEGIC PARTNERS. Part of our strategy involves building additional technology infrastructure to support our Universal Gene Recognition technology. This strategy includes the continued research and 8
12 development of improved and automated processes for design and production of our ZFP transcription factors. In addition, we intend to continue to assemble large collections, or libraries, of ZFPs for use in pharmaceutical target discovery. Because this infrastructure is an important part of our platform, any delay or failure to complete it could slow our growth and our ability to advance our strategic initiatives. OUR UNIVERSAL GENETOOLS COLLABORATION AGREEMENTS WITH COMPANIES ARE OF LIMITED SCOPE, AND IF WE ARE NOT ABLE TO EXPAND THE SCOPE OF OUR EXISTING COLLABORATIONS OR ENTER INTO NEW ONES, OUR REVENUES WILL BE NEGATIVELY IMPACTED AND OUR RESEARCH INITIATIVES MAY BE SLOWED OR HALTED. Our Universal GeneTools collaborations are important to us because they permit us to introduce our technology to many companies by supplying them with a specified ZFP transcription factor for a payment without licensing any of our technology. The collaboration agreements, however, are of limited scope. Under most of our current Universal GeneTools collaborations we receive a payment for supplying ZFP transcription factors for gene targets specified by the companies. These companies are not obligated to make continuing payments to us in connection with their research efforts or to pursue any product development program with us. As a result, we may not develop long-term relationships with these companies that could lead to additional revenues. If we are not able to expand the scope of our existing collaborations or enter into new ones, we may have reduced revenues and be forced to slow or halt research initiatives. COMMERCIALIZATION OF OUR TECHNOLOGIES DEPENDS ON STRATEGIC PARTNERING WITH OTHER COMPANIES, AND IF WE ARE NOT ABLE TO FIND STRATEGIC PARTNERS IN THE FUTURE, WE MAY NOT BE ABLE TO DEVELOP OUR TECHNOLOGIES OR PRODUCTS, WHICH COULD SLOW OUR GROWTH AND DECREASE OUR REVENUES. We expect to rely, to some extent, on our strategic partners to provide funding in support of our research and to perform some independent research, preclinical and clinical testing. We currently have only one strategic partner. Our technology is broad based and we do not currently possess the resources necessary to develop and commercialize potential products that may result from our technologies, or the resources or capabilities to complete any approval processes that may be required for the products, therefore we must enter into additional strategic partnerships to develop and commercialize products. Of the thousands of ZFP transcription factors which target specific genes, our current 18 collaborators and strategic partner are working with less than , therefore in order to fully utilize our ZFP transcriptions factors we would need a number of new Universal GeneTools collaborators and strategic partners to accomplish our research. We may require significant time to secure additional collaborations or strategic partners because we need to effectively market the benefits of our technology to these future collaborators and strategic partners, which uses the time and efforts of research and development personnel and our management. Further, each collaboration or strategic partnering arrangement will involve the negotiation of terms that may be unique to each collaborator or strategic partner. These business development efforts may not result in a collaboration or strategic partnership. If we do not enter into additional strategic partnering agreements, we will experience reduced revenues and may not develop or commercialize our products. The loss of our current or any future strategic partnering agreement would not only delay or terminate the potential development or commercialization of any products we may derive from our technologies but also delay or terminate our ability to test ZFP transcription factors for specific genes. If any strategic partner fails to conduct the collaborative activities successfully and in a timely manner, the preclinical or clinical development or commercialization of the affected product candidates or research programs could be delayed or terminated. 9
13 Our existing strategic partnering agreement is, and we would expect any future arrangement to be based on the achievement of milestones. Under the strategic partnering agreements, we expect to receive revenue for the research and development of a therapeutic product based on achievement of specific milestones. Achieving these milestones will depend, in part, on the efforts of our strategic partner as well as our own. In contrast, our current Universal GeneTools collaboration agreements only pay us to supply ZFP transcription factors for the collaborator's independent use, rather than for future results of the collaborator's efforts. If we or any strategic partner fails to meet specific milestones, then the strategic partnership can be terminated which could decrease our revenues. OUR UNIVERSAL GENETOOLS COLLABORATORS AND STRATEGIC PARTNERS MAY DECIDE TO ADOPT ALTERNATIVE TECHNOLOGIES OR MAY BE UNABLE TO DEVELOP COMMERCIALLY VIABLE PRODUCTS USING OUR TECHNOLOGY, WHICH WOULD NEGATIVELY IMPACT OUR REVENUES AND OUR STRATEGY TO DEVELOP THESE PRODUCTS. Our collaborators or strategic partners may adopt the alternative technology of our competitors which could decrease the marketability of our technology. Because many of our Universal GeneTools collaborators or strategic partners are likely to be working on more than one research project, they could choose to shift their resources to projects other than those they are working on with us. If they do so, that would delay our ability to test our technology and would delay or terminate the development of potential products based on our gene regulation technology. Further, our collaborators and strategic partners may elect not to develop products arising out of our collaborative and strategic partnering arrangements or to devote sufficient resources to the development, manufacturing, marketing or sale of these products. If any of these events occur, we may not be able to develop our technologies or commercialize our products. WE INTEND TO CONDUCT PROPRIETARY RESEARCH PROGRAMS TO DISCOVER THERAPEUTIC PRODUCT CANDIDATES. THESE PROGRAMS INCREASE OUR RISK OF PRODUCT FAILURE, MAY SIGNIFICANTLY INCREASE OUR RESEARCH EXPENDITURES, AND MAY INVOLVE CONFLICTS WITH OUR COLLABORATORS AND STRATEGIC PARTNERS. Conducting proprietary research programs may not generate corresponding revenue and may create conflicts with our collaborators or strategic partners. The implementation of this strategy will involve substantially greater business risks and the expenditure of significantly greater funds than our current research activities. In addition, these programs will require substantial commitments of time from our management and staff. Moreover, we have no experience in preclinical or clinical testing, obtaining regulatory approval or commercial-scale manufacturing and marketing of therapeutic products, and we currently do not have the resources or capability to manufacture therapeutic products on a commercial scale. In order for us to commercialize these products directly, we would need to develop, or obtain through outsourcing arrangements, the capability to execute all of these functions, market and sell products. We do not have these capabilities, and we may not be able to develop or otherwise obtain the requisite preclinical, clinical, regulatory, manufacturing, marketing and sales capabilities. In addition, disagreements with our Universal GeneTools collaborators or strategic partners could develop over rights to our intellectual property with respect to our proprietary research activities. Any conflict with our collaborators or strategic partners could reduce our ability to enter into future collaboration or strategic partnering agreements and negatively impact our relationship with existing collaborators and strategic partners, which could reduce our revenue and delay or terminate our product development. 10
14 BECAUSE IT IS DIFFICULT AND COSTLY TO PROTECT OUR PROPRIETARY RIGHTS, AND THIRD PARTIES HAVE FILED PATENT APPLICATIONS THAT ARE SIMILAR TO OURS, WE CANNOT ENSURE THE PROPRIETARY PROTECTION OF OUR TECHNOLOGIES AND PRODUCTS. Our commercial success will depend in part on obtaining patent protection of our technology and successfully defending these patents against third party challenges. The patent positions of pharmaceutical and biotechnology companies can be highly uncertain and involve complex legal and factual questions. No consistent policy regarding the breadth of claims allowed in biotechnology patents has emerged to date. Accordingly, we cannot predict the breadth of claims allowed in patents we own or license. We are a party to various license agreements that give us rights under specified patents and patent applications. We currently hold an exclusive sublicense for ZFP transcription factor technology which is limited to using the technology in human and animal healthcare. The scope of this license may be subject to dispute. We may need to license additional rights to commercialize our technology outside human and animal healthcare. We will seek to obtain a sublicense to these patent applications for use in our agricultural and industrial biotechnology efforts. If we are not able, however, to license these additional rights, it could harm our business. Similarly, our current licenses, and our future licenses will, contain performance obligations. If we fail to meet those obligations, the licenses could be terminated. If we are unable to continue to license these technologies on commercially reasonable terms, or at all, we may be forced to delay or terminate our product development and research activities. With respect to our present and any future sublicenses, since our rights derive from those granted to our sublicensor, we are subject to the risk that our sublicensor may fail to perform its obligations under the master license or fail to inform us of useful improvements in, or additions to, the underlying intellectual property owned by the original licensor. We are unable to exercise the same degree of control over intellectual property that we license from third parties as we exercise over our internally developed intellectual property. We generally do not control the prosecution of patent applications that we license from third parties; therefore, the patent applications may not be prosecuted in a timely manner. Others have filed and in the future are likely to file patent applications that are similar to ours. We are aware that there are academic groups and other companies that are attempting to develop technology which is based on the use of zinc finger and other DNA binding proteins, and that these groups and companies have filed patent applications. Several patents have been issued, although 11
15 Sangamo has no current plans to use the associated inventions. More particularly, we are aware of pending patent applications with claims directed to zinc finger libraries and methods of designing zinc finger DNA binding proteins. These applications are not issued patents. If the pending claims were granted in their present form, however, they could interfere with our right to commercialize our products and processes. If these or other patents issue, it is possible that the holder of any patent or patents granted on these applications may bring an infringement action against our collaborators, strategic partner or us claiming damages and seeking to enjoin commercial activities relating to the affected products and processes. The costs of litigating the claim could be substantial. Moreover, we cannot predict whether our Universal GeneTools collaborators, strategic partners or we would prevail in any actions. In addition, if the relevant patent claims were upheld as valid and enforceable and our products or processes were found to infringe the patent or patents, we could be prevented from making, using or selling the relevant product or process unless we could obtain a license or were able to design around the patent claims. While we believe that our proprietary intellectual property would give us substantial leverage to secure a cross-license, it is uncertain that any license required under that patent or patents would be made available on commercially acceptable terms, if at all. We believe that there may be significant litigation in the genomics industry regarding patent and other intellectual property rights which could subject us to litigation. If we become involved in litigation, it could consume a substantial portion of our managerial and financial resources. We have received unsolicited invitations to license existing patented technology from a number of third parties, at least one of which contained an allegation of infringement. Upon careful analysis of each of these technologies, we have determined that we already own rights to these technologies or that our scientific and commercial interests would not benefit from the acquisition of rights to these technologies. Further, we believe that the making, using or selling of our products and processes need not infringe any claims in the proffered patents. Accordingly, we have declined to enter into license negotiations with these parties. It is possible, however, that these parties will bring future actions against us, our Universal GeneTools collaborators or our strategic partners alleging infringement of their patents. As detailed above, the outcome of any litigation, particularly lawsuits involving biotechnology patents, is difficult to predict and likely to be costly regardless of the outcome. In these circumstances, the risks of a negative impact on our business can neither be clearly defined nor entirely eliminated. We rely on trade secrets to protect technology where we believe patent protection is not appropriate or obtainable. Trade secrets, however, are difficult to protect. While we require employees, academic collaborators and consultants to enter into confidentiality agreements, we may not be able to adequately protect our trade secrets or other proprietary information or enforce these confidentiality agreements. Our Universal GeneTools collaborators, strategic partners and scientific advisors have rights to publish data and information in which we may have rights. If we cannot maintain the confidentiality of our technology and other confidential information in connection with our collaborations and strategic partnerships, then we may not be able to receive patent protection or protect our proprietary information. See "Business -- Intellectual Property and Technology Licenses." OUR POTENTIAL THERAPEUTIC PRODUCTS ARE SUBJECT TO A LENGTHY AND UNCERTAIN REGULATORY PROCESS, AND IF THESE POTENTIAL PRODUCTS ARE NOT APPROVED, WE WILL NOT BE ABLE TO COMMERCIALIZE THOSE PRODUCTS. The Food and Drug Administration, or FDA, must approve any therapeutic and some diagnostic products based on ZFP technology before it can be marketed in the United States. The process for receiving regulatory approval is long and uncertain, and even if we had a potential product, this product may not withstand the rigors of testing under the regulatory approval processes. 12
16 Before commencing clinical trials in humans, we must submit and receive approval from the FDA of an Investigational New Drug Application. Clinical trials are subject to oversight by institutional review boards and the FDA and these trials must meet particular conditions, such that they: - must be conducted in conformance with the FDA's good clinical practice regulations; - must meet requirements for institutional review board oversight; - must meet requirements for informed consent; - are subject to continuing FDA oversight; - may require large numbers of test subjects; and - may be suspended by us or the FDA at any time if it is believed that the subjects participating in these trials are being exposed to unacceptable health risks or if the FDA finds deficiencies in the Investigational New Drug application or the conduct of these trials. We must also demonstrate that the product is safe and effective in the patient population that will be treated. Data obtained from preclinical and clinical activities are susceptible to varying interpretations that could delay, limit or prevent regulatory clearances. In addition, we may encounter delays or rejections based upon additional government regulation from future legislation or administrative action or changes in FDA policy during the period of product development, clinical trials and FDA regulatory review. Failure to comply with applicable FDA or other applicable regulatory requirements may result in criminal prosecution, civil penalties, recall or seizure of products, total or partial suspension of production or injunction, as well as other regulatory action against our potential products or us. Additionally, we have no experience in conducting and managing the clinical trials necessary to obtain regulatory approval. In addition, we may also require approval from the Recombinant DNA Advisory Committee, or RAC, which is the advisory board to the National Institutes of Health, or NIH, focusing on clinical trials involving gene transfer. We have not submitted an application with the FDA or any other regulatory authority for any product candidate, and neither the FDA nor any other regulatory authority has approved any therapeutic, diagnostic, agricultural or industrial product candidate developed with our technology for commercialization in the United States or elsewhere. REGULATORY APPROVAL, IF GRANTED, MAY BE LIMITED TO SPECIFIC USES OR GEOGRAPHIC AREAS WHICH COULD LIMIT OUR ABILITY TO GENERATE REVENUES. Regulatory approval may limit the indicated use for which we can market a product. Further, once regulatory approval for a product is obtained, it and its manufacturer are subject to continual review. Discovery of previously unknown problems with a product or manufacturer may result in restrictions on the product, manufacturer and manufacturing facility, including withdrawal of the product from the market. In Japan and Europe, regulatory agencies also set or approve prices. Even if regulatory clearance of a product is granted, this clearance is limited to those specific states and conditions for which the product is useful as demonstrated through clinical trials. We cannot ensure that any therapeutic product developed by us, alone or with others, will prove to be safe and effective in clinical trials and will meet all of the applicable regulatory requirements needed to receive marketing clearance. Outside the United States, our ability to market a product is contingent upon receiving a marketing authorization from the appropriate regulatory authorities so we cannot predict whether or 13
17 when we would be permitted to commercialize our product. These foreign regulatory approval processes include all of the risks associated with FDA clearance described above. LAWS OR PUBLIC SENTIMENT MAY LIMIT OUR PRODUCTION OF GENETICALLY ENGINEERED AGRICULTURAL PRODUCTS IN THE FUTURE, AND THESE LAWS COULD REDUCE OUR ABILITY TO SELL THESE PRODUCTS. Genetically engineered products are currently subject to public debate and heightened regulatory scrutiny, either of which could prevent or delay production of agricultural products. We may develop genetically engineered agricultural products for ourselves or with our strategic partners. The field testing, production and marketing of genetically engineered plants and plant products are subject to federal, state, local and foreign governmental regulation. Regulatory agencies administering existing or future regulations or legislation may not allow production and marketing of our genetically engineered products in a timely manner or under technically or commercially feasible conditions. In addition, regulatory action or private litigation could result in expenses, delays or other impediments to our product development programs or the commercialization of resulting products. The FDA currently applies the same regulatory standards to foods developed through genetic engineering as applied to foods developed through traditional plant breeding. Genetically engineered food products, however, will be subject to premarket review if these products raise safety questions or are deemed to be food additives. Governmental authorities could also, for social or other purposes, limit the use of genetically engineered products created with our gene regulation technology. Even if we are able to obtain regulatory approval of genetically engineered products, our success will also depend on public acceptance of the use of genetically engineered products including drugs, plants and plant products. Claims that genetically engineered products are unsafe for consumption or pose a danger to the environment may influence public attitudes. Our genetically engineered products may not gain public acceptance. The subject of genetically modified organisms has received negative publicity in Europe, which has aroused public debate. The adverse publicity in Europe could lead to greater regulation and trade restrictions on imports of genetically altered products. If similar adverse public reaction occurs in the United States, genetic research and its resulting products could be subject to greater domestic regulation and could decrease the demand for our technology and products. IF CONFLICTS ARISE BETWEEN US AND OUR COLLABORATORS, STRATEGIC PARTNERS, SCIENTIFIC ADVISORS OR DIRECTORS, THESE PARTIES MAY ACT IN THEIR SELF-INTEREST, WHICH MAY LIMIT OUR ABILITY TO IMPLEMENT OUR STRATEGIES. If conflicts arise between us and our corporate or academic collaborators, strategic partners or scientific advisors or directors, the other party may act in its self-interest which may limit our ability to implement our strategies. Some of our Universal GeneTools or academic collaborators or strategic partners are conducting multiple product development efforts within each area that is the subject of the collaboration with us. Generally, in each of our collaborations, we have agreed not to conduct independently, or with any third party, any research that is competitive with the research conducted under our collaborations. Our collaborations may cause us to limit the areas of research that we pursue, either alone or with others. Our collaborators or strategic partners, however, may develop, either alone or with others, products in related fields that are competitive with the products or potential products that are the subject of these collaborations. Competing products, either developed by the collaborators or strategic partners or to which the collaborators or strategic partners have rights, may result in their withdrawal of support for our product candidates. Some of our collaborators or strategic partners could also become competitors in the future. Our collaborators or strategic partners could develop competing products, preclude us from entering into collaborations with their competitors, fail to obtain timely regulatory approvals, terminate their 14
18 agreements with us prematurely or fail to devote sufficient resources to the development and commercialization of products. Any of these developments could harm our product development efforts. OUR COLLABORATIONS WITH OUTSIDE SCIENTISTS MAY BE SUBJECT TO CHANGE WHICH COULD LIMIT OUR ACCESS TO THEIR EXPERTISE. We work with scientific advisors and collaborators at academic research institutions. These scientists are not our employees and may have other commitments that would limit their availability to us. Although our scientific advisors generally agree not to do competing work, if a conflict of interest between their work for us and their work for another entity arises, we may lose their services. Although our scientific advisors and academic collaborators sign agreements not to disclose our confidential information, it is possible that some of our valuable proprietary knowledge may become publicly known through them. IF WE USE BIOLOGICAL AND HAZARDOUS MATERIALS IN A MANNER THAT CAUSES INJURY OR VIOLATES LAWS, WE MAY BE LIABLE FOR DAMAGES. Our research and development activities involve the controlled use of potentially harmful biological materials as well as hazardous materials, chemicals and various radioactive compounds. We cannot completely eliminate the risk of accidental contamination or injury from the use, storage, handling or disposal of these materials. In the event of contamination or injury, we could be held liable for damages that result, and any liability could exceed our resources. We are subject to federal, state and local laws and regulations governing the use, storage, handling and disposal of these materials and specified waste products. The cost of compliance with these laws and regulations could be significant. ANTI-TAKEOVER PROVISIONS IN OUR CERTIFICATE OF INCORPORATION AND DELAWARE LAW COULD PREVENT A POTENTIAL ACQUIROR FROM BUYING YOUR STOCK. Anti-takeover provisions of Delaware law, in our certificate of incorporation and equity benefit plans may make a change in control of our company more difficult, even if a change in control would be beneficial to our stockholders. These provisions may allow our board of directors to prevent or make changes in the management and control of our company. In particular, our board of directors will be able to issue up to 5,, shares of preferred stock with rights and privileges that might be senior to our common stock, without the consent of the holders of the common stock. Further, without any further vote or action on the part of the stockholders, the board of directors will have the authority to determine the price, rights, preferences, privileges and restrictions of the preferred stock. This preferred stock, if it is ever issued, may have preference over and harm the rights of the holders of common stock. Although the issuance of this preferred stock will provide us with flexibility in connection with possible acquisitions and other corporate purposes, this issuance may make it more difficult for a third party to acquire a majority of our outstanding voting stock. Similarly, our authorized but unissued common stock is available for future issuance without stockholder approval. In addition, our certificate of incorporation: - states that stockholders may not act by written consent but only at a stockholders' meeting; - establishes advance notice requirements for nominations for election to the board of directors or proposing matters that can be acted upon at stockholders' meetings; or - limits who may call a special meeting of stockholders. 15
19 RISKS RELATED TO THIS OFFERING OUR STOCK PRICE MAY BE VOLATILE, WHICH COULD RESULT IN SUBSTANTIAL LOSSES FOR INVESTORS PURCHASING SHARES IN THIS OFFERING. Volatility in the biotechnology market could cause you to incur substantial losses. Prior to this offering, you could not buy or sell our common stock publicly. An active public market for our common stock may not develop or be sustained after this offering. We will negotiate and determine the initial public offering price with the representatives of the underwriters based on several factors. In addition, the market price of our common stock may be highly volatile. The market prices of securities of biotechnology companies are currently highly volatile. The market price of our common stock may fluctuate significantly in response to the following factors, some of which are beyond our control: - changes in market valuations of similar companies, since many biotechnology companies have recently registered their securities to trade publicly and may create a more volatile trading sector; - announcements by us or our competitors of new or enhanced products, technologies or services or significant contracts, acquisitions, strategic relationships, joint ventures or capital commitments; - regulatory developments; - additions or departures of key personnel; - deviations in our results of operations from the estimates of securities analysts; and - future sales of our common stock or other securities. OUR STOCK PRICE COULD BE ADVERSELY AFFECTED BY ADDITIONAL SHARES BECOMING AVAILABLE FOR SALE. Sales of a substantial number of shares of our common stock, or the perception that these sales could occur, could depress the market price of our common stock and could impair our ability to raise capital through the sale of additional equity securities. In addition, we have entered into registration rights agreements with some investors that entitle these investors to have their shares registered for sale in the public market. The exercise of these rights could affect the market price of our common stock. See "Shares Eligible for Future Sale" for further information concerning potential sales of our shares after this offering. PURCHASERS IN THIS OFFERING WILL INCUR IMMEDIATE AND SUBSTANTIAL DILUTION. We expect that the initial public offering price of our common stock will be substantially higher than the book value per share of the outstanding common stock. As a result, you will incur immediate and substantial dilution of $ per share in the net tangible book value per share of common stock from the initial public offering price. In the past, we issued options and warrants to acquire common stock at prices significantly below the initial public offering price. The exercise of options and warrants currently outstanding could cause additional, substantial dilution to you. See "Dilution" for more detailed information regarding the potential dilution you may incur. INSIDERS WILL CONTINUE TO HAVE SUBSTANTIAL CONTROL OVER SANGAMO AFTER THIS OFFERING AND COULD DELAY OR PREVENT A CHANGE IN CORPORATE CONTROL. The interest of management could conflict with the interest of our other stockholders. Upon completion of this offering, our executive officers, directors and principal stockholders will beneficially 16
20 own, in the aggregate, approximately % of our outstanding common stock. As a result, these stockholders, if they choose to act together, will be able to exercise control over all matters requiring stockholder approval, including the election of directors and approval of significant corporate transactions. This could have the effect of delaying or preventing a change of control of Sangamo, which in turn could reduce the market price of our stock. SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS Some statements contained in this prospectus are forward-looking with respect to our operations, economic performance and financial condition. Statements that are forward-looking in nature should be read with caution because they involve risks and uncertainties, they are included, for example, in specific and general discussions about: - our strategy; - sufficiency of our cash resources; - revenues from existing and new collaborations; - product development; - our research and development and other expenses; - our operational and legal risks; and - our plans, objectives, expectations and intentions and any other statements that are not historical facts. Various terms and expressions similar to them are intended to identify these cautionary statements. These terms include: "anticipates," "believes," "continues," "could," "estimates," "expects," "intends," "may," "plans," "seeks," "should" and "will." Actual results may differ materially from those expressed or implied in those statements. Factors that could cause these differences include, but are not limited to, those discussed under "Risk Factors" and "Management's Discussion and Analysis of Financial Condition and Results of Operations." ABOUT THIS PROSPECTUS You should rely only on the information contained in this prospectus. We have not authorized anyone to provide you with information that is different from that contained in this prospectus. We are offering to sell, and seeking offers to buy, shares of common stock only in jurisdictions where offers and sales are permitted. The information contained in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or of any sale of common stock. This preliminary prospectus is subject to completion prior to this offering. Among other things, this preliminary prospectus describes our company as we currently expect it to exist at the time of this offering. Universal Gene Recognition(TM), Universal GeneTools(TM), ZFP-Diagnostics(TM), ZFP-Therapeutics(TM), ZFP-Transgenics(TM) and ZFP(TM) are our trademarks. We will apply to register Universal Gene Recognition, Universal GeneTools, ZFP-Diagnostics, ZFP-Therapeutics, ZFP-Transgenics and ZFP. All trademarks and trade names appearing elsewhere in this prospectus are the property of their respective holders. 17
21 USE OF PROCEEDS Our net proceeds from the sale of the 5,, shares of common stock we are offering will be approximately $ million, or $ million if the underwriters' over-allotment option is exercised in full, based on an assumed initial offering price of $ per share, after deducting the estimated underwriting discount and commissions and the estimated offering expenses. We currently expect to use the net proceeds of this offering for research and development, capital equipment and general corporate purposes. We may also use a portion of the net proceeds to acquire or invest in businesses, products and technologies that are complementary to our own, although no acquisitions are planned or being negotiated as of the date of this prospectus, and no portion of the net proceeds has been allocated for any specific acquisition or for acquisitions generally. Pending these uses, the net proceeds will be invested in short term, investment grade, interest-bearing securities. The principal purposes of the offering are to increase our capitalization and financial flexibility, to provide a public market for our common stock and to facilitate access to public equity markets. While it is not possible to estimate with certainty how the net proceeds of this offering will be used over the next three years, we believe that approximately $60 million will be used for research and development, approximately $10 million for capital equipment and the balance for general corporate purposes. Since these are only estimates, our management will have broad discretion in the application of net proceeds. DIVIDEND POLICY We have never paid dividends on our common or preferred stock. We currently intend to retain any future earnings to support the development of our business. Therefore, we do not currently anticipate paying any cash dividends in the foreseeable future. 18
22 CAPITALIZATION The following table sets forth our capitalization as of December 31, - on an actual basis - on a pro forma basis to give effect to: - automatic conversion of all outstanding shares of preferred stock into 9,, shares of common stock upon consummation of the offering; - the issuance of , shares of preferred stock in January which converts into , shares of common stock upon consummation of the offering; - the issuance of a $5 million note in January and a $ million note in March which convert, together with accrued interest, into , shares of common stock at an assumed initial public offering price upon consummation of the offering of $ - on a pro forma as adjusted basis to give effect to the sale of 5,, shares of our common stock at an assumed initial public offering price of $ per share in this offering, after deducting the estimated underwriting discounts and commissions and our estimated offering expenses. You should read this table with "Management's Discussion and Analysis of Financial Condition and Results of Operations" and the Financial Statements and Notes to the Financial Statements appearing elsewhere in this prospectus. AS OF DECEMBER 31, PRO FORMA ACTUAL PRO FORMA AS ADJUSTED (IN THOUSANDS) Long-term debt, less current portion $ $ $ Stockholders' equity: Preferred stock, $ par value, 6,, shares authorized, actual and pro forma, 5,, shares authorized, as adjusted; 4,, shares issued and outstanding, actual, no shares issued and outstanding, pro forma and pro forma as adjusted 15, -- -- Common stock, $ par value, 15,, authorized, actual, 80,, shares authorized, pro forma and pro forma as adjusted; 6,, shares issued and outstanding, actual, 17,, shares issued and outstanding, pro forma and 22,, shares issued and outstanding, pro forma as adjusted 3, 32, , Note receivable from stockholder () () () Deferred stock compensation (1,) (1,) (1,) Accumulated deficit (8,) (8,) (8,) Accumulated other comprehensive income 83 83 83 Total stockholders' equity 7, 21, 95, Total capitalization $ 8, $22, $ 95, ======= ======= ======== The number of shares of common stock outstanding excludes: - 1,, shares of common stock issuable upon exercise of stock options outstanding at a weighted average exercise price of $ per share; - , shares of common stock issuable upon the exercise of outstanding warrants at a weighted average exercise price of $ per share; and - a total of 2,, shares of common stock available for future issuance under our stock option plans. 19
23 DILUTION Our pro forma net tangible book value at December 31, was $ million, or $ per share, assuming the conversion of our preferred stock into common stock upon consummation of the offering. Pro forma net tangible book value per share represents total net tangible assets less liabilities, divided by pro forma common shares outstanding after giving effect to the conversion of our preferred stock into common stock upon the consummation of this offering. Subsequent to December 31, , we issued , shares of preferred stock for $ million which converts into , shares of common stock upon consummation of this offering, and a $5 million note in January and a $ million note in March which convert, together with accrued interest, into , shares of common stock at an assumed initial offering price of $, upon consummation of this offering. These subsequent issuances increased our pro forma net tangible book value per share by $, assuming their conversion into common stock. After giving effect to our sale of shares of common stock in this offering and after deducting the underwriting discounts and commissions and our estimated offering expenses, our pro forma net tangible book value as of December 31, would have been $ million, or $ per share. This represents an immediate increase in pro forma net tangible book value of $ per share to existing stockholders and an immediate dilution of $ per share to new investors. Dilution in pro forma net tangible book value per share represents the difference between the amount per share paid by purchasers of shares of our common stock in this offering and the pro forma net tangible book value per share of our common stock immediately following this offering. The following table illustrates this per share dilution: Initial public offering price per share $ Pro forma net tangible book value per share at December 31, $ Increase per share attributable to equity and convertible note issuances subsequent to December 31, Increase per share attributable to the offering Pro forma net tangible book value per share after the offering Dilution per share to new investors $ ====== The following table summarizes, using the same pro forma assumptions as above and assuming an initial public offering price of $, the differences between the existing stockholders and new investors with respect to the number of shares of common stock purchased from us, the total consideration paid to us, and the average price per share. SHARES PURCHASED TOTAL CONSIDERATION AVERAGE PRICE NUMBER PERCENT AMOUNT PERCENT PER SHARE Existing stockholders 17,, 78% $ 29,, 27% $ New investors 5,, 22 80,, 73 Totals 22,, % $,, % ========== === ============ === - This table excludes the following shares as of December 31, - 1,, shares issuable upon exercise of outstanding options at a weighted average exercise price of $ per share; - , shares issuable upon exercise of outstanding warrants at a weighted average exercise price of $ per share; and - a total of 2,, shares available for future issuance under our stock plans. See "Management -- Stock Plans" and Note 4 of Notes to Financial Statements. 20
24 SELECTED FINANCIAL DATA Our audited financial statements, which have been audited by Ernst & Young LLP, were used for the following selected statement of operations data for the period from inception to December 31, and for the years ended December 31, , , and , and the balance sheet data as of December 31, , , , and The diluted net loss per share computation excludes potential shares of common stock (preferred stock, options and warrants to purchase common stock and common stock subject to repurchase rights that we hold), since their effect would be antidilutive. See Note 1 of Notes to Financial Statements for a detailed explanation of the determination of the shares used to compute actual and pro forma basic and diluted net loss per share. Our historical results are not necessarily indicative of results to be expected for future periods. You should read the following selected financial data along with our Financial Statements and related Notes and "Management's Discussion and Analysis of Financial Condition and Results of Operations" included elsewhere in this prospectus. YEAR ENDED DECEMBER 31, (IN THOUSANDS, EXCEPT PER SHARE DATA) STATEMENT OF OPERATIONS DATA: Total revenues $ $ $ 1, $ 2, $ 2, Operating expenses: Research and development 1, 4, 4, General and administrative 50 1, 1, Total operating expenses 2, 5, 6, Loss from operations (17) () (1,) (3,) (3,) Interest income (expense), net -- 10 (55) Net loss $ (17) $ () $(1,) $(3,) $(3,) ====== ====== ======= ======= ======= Basic and diluted net loss per share $() $() $ () $ () $ () ====== ====== ======= ======= ======= Shares used in computing basic and diluted net loss per share 5, 5, 5, 5, 5, ====== ====== ======= ======= ======= Pro forma basic and diluted net loss per share (unaudited) $ () ======= Shares used in computing pro forma basic and diluted net loss per share (unaudited) 13, ======= AS OF DECEMBER 31, (IN THOUSANDS) BALANCE SHEET DATA: Cash, cash equivalents and short-term investments $ $ $ 6, $ 3, $ 7, Working capital 6, 3, 7, Total assets 6, 4, 9, Long-term debt -- -- -- Accumulated deficit (17) () (1,) (5,) (8,) Total stockholders' equity 6, 3, 7, 21
25 MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS You should read the following discussion and analysis along with the "Selected Financial Data" and the financial statements and notes attached to those statements included elsewhere in this prospectus. OVERVIEW We were incorporated in June From our inception through December 31, , our activities related primarily to establishing a research and development organization and developing relationships with our Universal GeneTools collaborators. We have incurred net losses since inception and expect to incur losses in the near future as we expand our research and development activities. To date, we have funded our operations primarily through the issuance of equity securities, borrowings, and payments from federal government research grants and from Universal GeneTools collaborators. As of December 31, , we had an accumulated deficit of $ million. Our revenues consist primarily of federal government research grant funding and revenues from our Universal GeneTools collaborators. We expect that in the near future, our revenues will also include payments from strategic partners for technology access fees, committed research funding and research milestone payments. In January , we announced that we had entered into a strategic partner agreement with Edwards LifeScience, Inc., formerly the CardioVascular Group of Baxter Healthcare Corporation for the development of ZFPs in cardiovascular and peripheral vascular diseases. Under this agreement, Baxter has purchased a $5 million convertible note which will convert, together with accrued interest, into common stock upon consummation of this offering, and we have received $1 million in initial research funding from Baxter. In March , Baxter exercised an option by purchasing a $ million convertible note, which will convert, together with accrued interest, into common stock upon consummation of this offering, for a right of first refusal to negotiate a license for additional ZFP-Therapeutics in cardiovascular and peripheral vascular disease. In the future, we may receive option fees, milestone payments, royalties and additional research funding from this agreement. See "Business -- Corporate Collaborations" and Note 7 of Notes to Financial Statements. Research and development expenses consist primarily of salaries and related personnel expenses, subcontracted research expenses, and technology license expenses. As of December 31, , all research and development costs have been expensed as incurred. We believe that continued investment in research and development is critical to attaining our strategic objectives. We expect these expenses will increase significantly in the future as we continue to develop our Universal Gene Recognition technology platform. General and administrative expenses consist primarily of salaries and related personnel expenses for executive, finance and administrative personnel, professional fees, and other general corporate expenses. As we add personnel and incur additional costs related to the growth of our business, general and administrative expenses will also increase. STOCK COMPENSATION During the years ended December 31, , and , in connection with the grant of stock options to employees and directors, we recorded deferred stock compensation totaling $,, $, and $ million, respectively, representing the difference between the fair value of our common stock on the date such options were granted and the exercise price. These amounts are 22
26 included as a reduction of stockholders' equity and are being amortized over the vesting period of the individual options, generally four years, using the graded vesting method. The graded vesting method provides for vesting of portions of the overall award at interim dates and results in higher vesting in earlier years than straight-line vesting. The fair value of our common stock for purposes of this calculation was determined based on the business factors underlying the value of our common stock on the date such option grants were made, viewed in light of this offering and the expected initial public offering price per share. We recorded amortization of deferred stock compensation of $46,, $, and $,, for the years ended December 31, , and , respectively. At December 31, , we had a total of $ million remaining to be amortized over the vesting periods of the stock options. Through March 13, we recorded additional deferred stock compensation of $ million in connection with grants of stock options subsequent to December 31, and we may record additional deferred stock compensation for options granted prior to the closing of this offering. You should read Note 4 of Notes to Financial Statements for more information. RESULTS OF OPERATIONS Years Ended December 31, and Total revenues. Total revenues consist of revenues from collaboration agreements and federal government research grants. Revenues from our Universal GeneTools agreements were $ million in , compared with $, during , an increase of $, The increase in was principally attributable to revenues recognized from collaboration agreements signed since the third quarter of We expect revenues from these agreements to continue to increase as additional agreements are signed or existing agreements are expanded. Federal government research grant revenues were $ million in , compared to $ million in , a decrease of $, The decrease in was principally due to an increased focus on Universal GeneTools collaborations and strategic partners in as some existing federal research government grants ended. We plan to continue to apply for federal government research grants. Research and development expenses. Research and development expenses were $ million for and as reductions in laboratory supplies and equipment expenses were offset by increases in stock compensation expense. We expect research and development expenses to increase significantly in future periods, particularly as we increase the scientific staff to continue to develop the Universal Gene Recognition technology and to meet the needs of our Universal GeneTools collaborators and strategic partners. General and administrative expenses. General and administrative expenses increased by $,, from $ million in to $ million in This increase was primarily attributable to increased staffing to support our expanded research and development activities and development of our Universal Gene Recognition technology. We expect that general and administrative expenses will increase in the future to support continued growth of our research and development efforts. Interest income (expense), net. Interest income (expense), net decreased by $42, from $, in to $, in The decrease in interest income, net resulted from lower average interest-bearing balances and higher debt balances during Years Ended December 31, and Total revenues. Federal government research grant revenues increased by $, from $ million in to $ million in This increase was principally attributable to revenue from new federal government research grants, including a grant from the Department of Commerce under the Advanced Technology Program initiated in late 23
GCI Combined Services (CS)
Exhibit
* Confidential Portion has been omitted pursuant to a request for confidential treatment by the Company to, and the material has been separately filed with, the SEC. Each omitted Confidential Portion is marked by three Asterisks.
TWELFTH AMENDMENT TO CONTRACT FOR ALASKA ACCESS SERVICES
This TWELFTH AMENDMENT TO THE CONTRACT FOR ALASKA ACCESS SERVICES (&#;Twelfth Amendment&#;) is entered into and effective as of the 13th&#;&#; day of December, (&#;Effective Date&#;), by and between GENERAL COMMUNICATION, INC. and its indirectly, wholly-owned subsidiary, GCI COMMUNICATION CORP., both Alaska corporations(together, &#;GCI&#;) with offices located at Denali Street, Suite , Anchorage, Alaska and MCI COMMUNICATIONS SERVICES, INC., d/b/a Verizon Business Services (successor-in-interest to MCI Network Services, Inc., which was formerly known as MCI WORLDCOM Network Services (&#;Verizon&#;), with offices located at 19th Street, N.W., Washington, D.C. (GCI with Verizon, collectively, the &#;Parties,&#; and individually, a &#;Party&#;).
RECITALS
WHEREAS,
WHEREAS, the General Services Administration (&#;GSA&#; or &#;Customer&#;) issued Networx Universal and Enterprise Solicitations for Telecommunication Services (&#;the Project&#;); and
WHEREAS, the Parties have agreed to incorporate into this Agreement the terms and conditions of the Teaming Agreement dated as of July 14, , as amended by Amendment No. 1 dated as of March 23, , both between GCI Communication Corp. and Verizon Services Corp., (collectively, the &#;Teaming Agreement&#;), wherein the Parties provide complementary talents, experience and capabilities to respond to the solicitations for and to perform telecommunications services for the Project, as described below.
1
AGREEMENT
NOW,THEREFORE, for good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the Parties agree as follows:
The above recitals are incorporated into the Agreement.
I.&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; Section 4.A.(2) of the Agreement is hereby revised to read as follows:&#; &#;The Party shall have failed to perform its obligations under the Agreement, coupled with a failure to remedy nonperformance within thirty days after receipt of written notice thereof from the other Party.&#;
II. Section 4.C. of the Agreement, as previously amended, is hereby deleted in its entirety and replaced with the following:
&#;C. DISPUTE RESOLUTION.
Any dispute, controversy or claim (collectively a &#;Dispute&#;) arising out of or relating to this Agreement will ***. Should resolution not occur ***, the Dispute will ***. If the Dispute cannot be resolved in good faith ***, the Parties may exercise any and all available remedies at law or equity (including injunctive relief) or may request that the Dispute be settled by binding arbitration. In the event the Parties agree to binding arbitration, which agreement shall be at their sole and absolute discretion, the costs of arbitration, including fees and expenses of the arbitrator, shall be shared equally by the Parties and each Party shall bear the cost of preparing its case.&#;
III.&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; Section 5 MISCELLANEOUS of the Agreement shall be hereby re-numbered to be Section 15 MISCELLANEOUS.
IV.&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; As of the Effective Date of this Twelfth Amendment, new Sections 5, 6, 7, 8, 9, 10, 11, 12,&#; 13 and 14 of the Agreement shall be added as follows:
&#;5.&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; TEAMING ACTIVITIES.
A.&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; With the assistance ***, and subject to any necessary management approvals, *** proposal to the Customer for the Project. *** in the proposal as the prime contractor for the Project. *** proposal as a proposed subcontractor *** with the Project as generally described in the Exhibit As, attached to this Agreement and incorporated herein by this reference, and for the price(s) and/or fee(s) asset forth in Exhibit F hereto. *** acknowledges *** pre-award OSS Testing, Product Testing and Certification and Acceptance Testing as required by the Customer. *** and at no additional cost to ***.
B.&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; *** with all reasonable assistance in the development and preparation of any proposal(s) that may be required, including any best and final offer(s). The *** content of any *** the Customer ***. *** will include *** in its proposal(s) *** furnished ***. In its sole discretion, *** the provisions of the proposal.
2
C.&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; Both Parties will make available their respective management and technical personnel as may be appropriate during the conduct of any discussions and negotiations with the Customer concerning the award of a prime contract for the Project to ***.
D.&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; Each Party hereby authorizes the other Party to use any information, data or drawings provided hereunder consistent with Section 10, PROPRIETARY INFORMATION, solely for the express purpose of developing and presenting the Project proposal and obtaining a prime contract award to *** for the Project (the &#;Purpose&#;).
6.&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; ALLOCATION OF COSTS.
Each Party will be responsible for and bear the cost of its own efforts in the preparation and support of its portion of the proposal requirements and other responsibilities set forth in this Agreement.
7.&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; INDEPENDENT CONTRACTORS.
This Agreement is not intended to constitute, create, give effect or otherwise recognize a joint venture, partnership, principal-agent or formal business organization of any kind, and the rights and obligations of the Parties shall be only those expressly set forth herein. At all times Verizon and GCI shall remain independent contractors, each responsible for its own employees. Neither Party assumes responsibility to the other for costs, expenses, risks and liabilities arising out of the efforts of the other Party under this Agreement.
8.&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; TEAMING AGREEMENT.
A.&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; Nothing contained in this Agreement shall be deemed to restrict either Party from quoting, offering to sell or selling to others any items or services that it may regularly offer for sale or license, even though such items or services may be included in the Project proposal contemplated by this Agreement. Notwithstanding the nature of the Parties&#; relationship under this Agreement, the Parties do not intend to prejudice the Customer in any way with respect to any action that it may take in procuring goods or services on the basis of competitive proposals or the awarding of contracts on a split or other type basis.
B.&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; The Parties will continue to follow procedures established by and known to the Parties for the ordering, provisioning and inventory of Services until such time that the Parties further amend the Agreement with regard thereto by adding Exhibits H and I to the Agreement.
9.&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; SUBCONTRACT.
In the event *** is awarded a prime contract for the Project as a result of the proposal contemplated by this Agreement, and this Agreement or the provisions hereof relating to the Project have not been previously terminated pursuant to the applicable provisions hereof, *** negotiations with *** intended to culminate in a further amendment hereto consistent with this Agreement and the Exhibits hereto, to the extent the same is required in the prime contract, and
3
further subject to: (a) any necessary approvals by the Customer; (b) the inclusion of any additional necessary or appropriate &#;flow-down&#; clauses and other provisions from the prime contract between *** and Customer as *** deems necessary in its sole discretion to enable it to comply with its prime contract obligations and to provide *** with the required control over *** of the Project; and (c) the negotiation of other mutually acceptable terms and conditions. *** acknowledges that it has read and understands the Solicitation and all amendments thereto issued prior to the Effective Date hereof.
&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; Section 15 D, as renumbered herein, is hereby deleted in its entirety and replaced with the following:
D. PROPRIETARY INFORMATION.
If in the course of performing this Agreement, either Party discloses any proprietary or confidential information to the other Party, such disclosure shall be governed by the following provisions:
A.&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; For purposes of this Agreement, the term &#;Proprietary Information&#; means all information (i) related to the Agreement or the Project that the Parties may exchange under this Agreement or otherwise for the Purpose stated in Section 5 of the Agreement or (ii) that, although not related to the Agreement or such Purpose, is nevertheless disclosed by a Party (&#;Owner&#;) to the other Party (&#;Recipient&#;) as a result of the Parties&#; discussions in that regard, and is identified to be proprietary and confidential to the Owner, an Affiliate of the Owner or to a third party. The term &#;Affiliate&#; means any person or entity directly or indirectly controlling, controlled by, or under common control with a Party. Proprietary Information disclosed in written or other tangible form (including on magnetic media) shall be marked with legends that identify the information as confidential or proprietary. Proprietary Information disclosed by oral, visual or other non-tangible means shall be reasonably identified as such by Owner at the time of disclosure. Notwithstanding the foregoing, and except as provided in subsection (d) below, the following categories of information shall be deemed to be Proprietary&#;&#; Information in all cases, regardless of the form of disclosure or identification or lack of identification by a Party: proprietary network engineering designs; software; pricing and financial information; names of clients; and/or future product offerings.
B.&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; Proprietary Information shall be used by the Recipient only in the performance contemplated by this Agreement and shall not be disclosed to any other person, firm, corporation or partnership or used for any other purpose than the Purpose contemplated by this Agreement without the prior written consent of the Owner.
C.&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; To protect the Owner&#;s Proprietary Information from unauthorized use or disclosure, Recipient shall use the same degree of care that it uses to prevent unauthorized use or disclosure of its own proprietary or confidential information, data or drawings of like importance, but in no event shall the Recipient use less than a reasonable degree of care.
D.&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; The obligation to protect Proprietary Information as set forth in this Agreement, shall not apply to any of the following:
4
(1)&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; Information that is known to the Recipient without restriction when received, or thereafter is developed independently by the Recipient; or
(2)&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; Information that was obtained by Recipient from a source other than the Owner through no breach of confidence by the Recipient; or
(3)&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; Information that was in the public domain when received, or thereafter enters the public domain through no fault of the Recipient; or
(4)&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; Information that was disclosed by the Owner to a third party without restriction.
E.&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; GCI&#; hereby grants its consent for Verizon to disclose GCI&#;s Proprietary Information to the Customer, provided that Verizon shall identify any GCI Proprietary Information disclosed to the Customer as proprietary and shall request that Customer&#;s use of any such GCI Proprietary Information be restricted to use in reviewing and evaluating Verizon&#;s Project proposal as contemplated by this Agreement.
F.&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; Each Party&#;s obligations hereunder concerning Proprietary Information received from the other Party shall ***, notwithstanding any earlier expiration or termination of this Agreement. Upon expiration or termination of this Agreement, each Party shall cease use of Proprietary Information received from the other Party, and shall destroy all such Proprietary Information, including copies thereof, then in its possession or control, promptly furnishing the Owner with written certification of such destruction. Alternatively, at the request of the Owner, the Recipient shall return all such Proprietary Information and copies to the Owner. The rights and obligations of the Parties under this Section 15 shall survive any such return or destruction of Proprietary Information.
G.&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; The Parties agree that, in the event of a breach or threatened breach of the terms of this Section 15, the Owner shall be entitled to seek an injunction or other equitable relief prohibiting any such breach, without the necessity of posting a bond. Any such relief shall be in addition to and not in lieu of any appropriate relief in the way of money damages. The Parties acknowledge that Proprietary Information is valuable and unique and that unauthorized use or disclosure in breach of this Section 15 could result in irreparable injury to the Owner.
H.&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; All Proprietary Information received hereunder (including information in computer software or held in electronic storage media) shall remain the property of the Owner. Nothing contained in this Agreement, nor any disclosure hereunder, shall be construed as a grant of any right or license, express or implied, under any patent, copyright or other intellectual property right of the Owner.
&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; LIABILITY.
A.&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; Each Party will be solely responsible for liability arising out of its own acts or omissions occurring during the performance of its work under this Agreement. The performing
5
Party further agrees to indemnify, hold harmless and defend the other from all costs of any nature whatsoever arising out of any third party claim or action against the other Party resulting from the acts or omissions of the performing Party. The indemnified Party shall: (i) provide written notice of the claim or action to the indemnifying Party promptly after becoming aware of the same, (ii) relinquish control of the defense and/or settlement of such action or claim to the indemnifying Party, and (iii) at the indemnifying Party&#;s request, provide reasonable assistance and cooperation to the indemnifying Party, at the indemnifying Party&#;s sole expense. The indemnified Party&#;s failure to comply with any of the foregoing shall not modify any of indemnifying Party&#;s obligations under this section except to the extent that the indemnifying Party&#;s ability to fulfill any of such obligations is materially prejudiced by such failure. This provision shall not be construed to mean that the Parties are precluded from resolving a claim against each other.
B.&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; In the event of an alleged breach of this Agreement, or any claim whether in tort (including negligence and strict liability), contract, equity or otherwise, arising out of or in connection with this Agreement, or the acts or omissions of either Party, its agents, representatives or employees in the performance of this Agreement, the Parties agree that the sole remedy available shall be limited to the recovery of direct costs and applicable overhead reasonably expended in performance of the services related to the Project.
C.&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; In no event shall either Party be liable to the other Party for any special, indirect, incidental, punitive or consequential damages, including but not limited to lost profits or revenue, or lost business opportunities, even if advised of the possibility of such damages.
&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; INSURANCE.
GCI shall maintain for the Term of the Agreement the following insurance:
A.&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; Commercial General Liability Insurance, on an occurrence basis, including but not limited to, premises-operations, broad form property damage, products/completed operations, contractual liability, independent contractors, and personal injury, with limits of at least $*** combined single limit for each occurrence.
B.&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; Commercial Automobile Liability, with limits of at least $*** combined single limit for each occurrence.
C.&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; Excess Liability, in the umbrella form, with limits of at least $*** combined single limit for each occurrence.
D.&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; Workers&#; Compensation Insurance as required by Applicable Law and Employer&#;s Liability Insurance with limits of not less than $***.
E.&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; All risk property insurance policy to cover GCI&#;s property, furnishings, & equipment.
F.&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; The above limits may be satisfied by a combination of underlying/primary and excess/umbrella insurance. All policies provided by GCI shall be ***. GCI shall waive its right
6
of subrogation for all insurance claims. The Commercial General Liability and Commercial Auto Liability policies must name Verizon, its subsidiaries and affiliates as additional insureds. GCI&#;s insurance companies must be licensed to do business in the applicable state(s) and must meet or exceed an A.M. Best rating of A-X or its equivalent.
G.&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; All insurance must be in effect ***. For all insurance, GCI must deliver an industry-recognized certificate of insurance evidencing the amount and nature of the coverage, the expiration date of the policy and the waiver of subrogation and stating that the policy of insurance issued to GCI ***. Also, where applicable, such certificate of insurance shall evidence the name of Verizon as an additional insured. GCI shall submit such certificates of insurance annually to Verizon as evidence that it has maintained all required insurance. GCI is responsible for determining whether the above minimum insurance coverages are adequate to protect its interests. The above minimum coverages shall not constitute limitations upon GCI&#;s liability.
&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; TERMINATION OF TEAMING ACTIVITIES.
Only Sections 5, 6, 8 and 9 of this Agreement, and all rights and obligations of each Party thereunder, shall automatically terminate upon the happening of any of the following:
A.&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; *** the Project or the *** the Customer, or *** the Customer of ***&#; under the Project allocated to *** under Exhibit A hereto;
B.&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; The Customer&#;s *** for the Project to a contractor other than ***;
C.&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; The Parties&#; execution *** to perform the work allocated *** in Exhibit A hereto;
D.&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; The Customer&#;s refusal *** for the portion of the Project *** in the Exhibit As hereto, despite *** reasonable efforts to secure such approvals;
E.&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; Upon notice from *** to *** if, as a result of judicial action or ruling, *** or the Customer is prohibited from utilizing *** to perform the work allocated ***&#; in Exhibit A hereto, or is required to utilize a different subcontractor to perform the work allocated to *** in the&#; Exhibit As hereto;
F.&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; Lapse of ***, unless such term is extended by mutual agreement;
G.&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; Mutual agreement of the Parties to terminate Sections 5, 6, 8 and 9 of this Agreement; or
H.&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; A material, uncured breach by either Party of any of the provisions contained herein, as described in Section 4 hereof.
&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; PUBLICITY.
Any news release (including communication of any sort with the press whether direct or
7
indirect, written or oral), public announcement or advertisement to be released in connection with this Agreement and the subject matter hereunder shall have the written concurrence of both Parties prior to release. The provisions of this Section 14 shall survive the termination or expiration of this Agreement.&#;
V.&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; NOTICES.
Section C. of the Agreement is hereby deleted in its entirety and replaced with the following:
&#;All notices required or permitted to be given hereunder shall be in writing and be deemed effective (a) upon personal delivery, (b) on the calendar day following the date of confirmed transmission of telex, telegram, or electronic mail, or (c) upon receipt if sent by registered, certified or express mail to the Parties addressed as follows:
If to Verizon: | Verizon | |
| Old Gallows Rd. | |
| Vienna, VA. | |
|
| |
| Attn: |
|
|
| |
With a contemporaneous copy to: | Verizon Purchasing LLC. (MCI) | |
| Old Gallows Rd. | |
| Vienna, VA | |
|
| |
| Attn: |
|
|
| |
If to GCI: | GCI Communication Corp. | |
| Denali Street, Suite | |
| Anchorage, AK | |
| Attn: Richard Westlund, | |
| Senior Vice President & General Manager, | |
| Network Access Services | |
|
| |
With a contemporaneous copy to: | GCI Communication Corp. | |
| Denali Street, Suite | |
| Anchorage, AK | |
| Attn: Corporate Counsel | |
Either Party may change the address or addressee set forth above at any time or times, by &#;written notice to the other Party in accordance with this provision.&#;
VI.&#;&#;&#;&#;&#;&#;&#;&#;&#; As of the Effective Date of this Twelfth Amendment, Section F. shall be deleted and replaced in its entirety with the following:
&#;F. Binding Effect and Assignment. This Agreement shall be binding upon and shall inure to the benefit of the Parties and their respective permitted successor and assigns. Neither
8
Party may assign, delegate, or transfer any part of this Agreement without the other Party&#;s prior written consent, which shall not be unreasonably withheld, except that either Party may assign this Agreement in part or whole&#; to an Affiliate. An affiliate for this purpose in an entity controlling, controlled by or under common control with the assigning party. Any attempted assignment not conforming with this provision shall be void.&#;
VII.&#;&#;&#;&#;&#;&#;&#;&#; New Attachments. All of the following are hereby incorporated into the Agreement:
Exhibit A &#; Scope of Work
Exhibit B &#; Performance and Maintenance
Exhibit C &#; Networx Prime Contract &#;Flow Down&#;, provisions for Subcontractor
Exhibit D &#; FAR Provisions
Exhibit E &#; Technical Specification
Exhibit F &#; Pricing
Exhibit G &#; ICD &#; Billing Feed
Exhibit H &#; ICD &#; Order and Inventory&#; (Reserved)
Exhibit I &#; ICD &#; Manual ASR&#; (Reserved)
VIII.&#;&#;&#;&#;&#;&#; Effect of Amendment. All other terms and conditions of the Agreement not expressly modified by this Twelfth Amendment shall remain in full force and effect. The Parties hereby affirm and agree such terms remain binding.
IX.&#;&#;&#;&#;&#;&#;&#;&#;&#; Further Assurances. The Parties shall cooperate in good faith, and enter into such other instruments and take such other actions, as may be necessary or desirable, to fully implement the intent of this Twelfth Amendment.
X.&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; Severability If any provision of this Agreement or any Service Order is found to be invalid or unenforceable, it shall not affect the validity and enforceability of any other provision of the Agreement, and the invalid or unenforceable provision shall be curtailed or limited only to the extent necessary to permit compliance with the minimum legal requirements, in a manner as consistent as possible with the intentions of the Parties and the economic position contemplated in the Agreement.
XI.&#;&#;&#;&#;&#;&#;&#;&#;&#; Entire Agreement. This Twelfth Amendment, together with the Agreement, including exhibits hereto and other documents incorporated herein by reference, contains the complete agreement of the Parties with regard to the subject matter herein and supersedes and replaces all other prior contracts and representations concerning its subject matter. In the event of a conflict between the terms of this Twelfth Amendment and the Agreement, the terms of this Twelfth Amendment shall control. Any further amendments to the Agreement must be in writing and signed by authorized representatives of both Parties.
IN WITNESS WHEREOF, the Parties hereto each acting with proper authority have executed this Twelfth Amendment as of the Effective Date first above written.
9
MCI COMMUNICATIONS SERVICES, INC. | |||||||
| |||||||
By: | /s/ | Peter H. Reynolds |
| ||||
|
| ||||||
Printed Name: | Peter H. Reynolds |
| |||||
|
| ||||||
Title: | Director |
| |||||
|
| ||||||
|
| ||||||
GCI COMMUNICATION CORP. |
| ||||||
|
| ||||||
By: | /s/ Richard Westlund |
| |||||
Printed Name: Richard Westlund |
| ||||||
Title: Senior Vice President & General Manager, Network Access Services | |||||||
|
| ||||||
GENERAL COMMUNICATION, INC. |
| ||||||
|
| ||||||
|
| ||||||
By: | /s/ Richard Westlund |
| |||||
Printed Name: Richard Westlund |
| ||||||
Title: Senior Vice President & General Manager, Network Access Services | |||||||
10
Exhibit A
SCOPE OF WORK
COMBINED SERVICES
(1 OF 14)
11
STATEMENT OF WORK
&#;&#;&#;&#;&#;&#;&#;&#;&#; Objective
Verizon has selected GCI to meet the requirements for Combined Services, limited to Alaska, (CS). GCI will comply with all requirements for C as outlined on the following pages.
Combined Services (CS) is a collection of separate telecommunications services packaged into a single service offering from a contractor. Agencies may utilize a Combined Services package to provide a core telecommunications service that suits their fundamental business needs.
&#;&#;&#;&#;&#;&#;&#;&#;&#; Background
GCI shall offer a technical solution which meets the requirements of GSA&#;s Networx Universal and Enterprise RFPs (heretofore referred to as Networx RFPs) Section C, Networx Combined Services, as well as provide pricing in the structure provided for in Section B of the Networx RFPs, limited to Alaska. If GCI does not provide any of the services described in Section C, then it will be GCI&#;s responsibility to identify a teaming partner or solution to meet all of the requirements in Section C as it relates to Networx Combined Services, as it pertains to Alaska coverage.
&#;&#;&#;&#;&#;&#;&#;&#;&#; Project Scope
GCI is only providing the local and Alaska LD for this service. VZB is providing the long distance portion of the service.
Technical Requirements
Section C of Networx Universal RFP
GCI shall offer a technical solution which meets the requirements of GSA&#;s Networx Universal and Enterprise RFPs (heretofore referred to as Networx RFPs) Section C, Networx Combined Services, as well as provide pricing in the structure provided for in Section B of the Networx RFPs, limited to Alaska. If GCI does not provide any of the services described in Section C, then it will be GCI&#;s responsibility to identify a teaming partner or solution to meet all of the requirements in Section C as it relates to Networx Combined Services, as it pertains to Alaska coverage. This includes:
&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; Providing CS Service transport between the subscribing Networx Agency&#;s Service Delivery Point (SDP) and the GCI trunking interface point to Verizon&#;s POP in Seattle.
12
&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; Installing and maintaining Service Enabling Device(s) that may be ordered for the subscribing Agency&#;s SDP.
&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; Responding to Verizon trouble tickets reported to Verizon&#;s Help Desk.
&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#;&#; Providing SLA and KPI performance data shown in RFP Sections J and C respectively.
What’s New in the Zip Express 2.7.2.1 serial key or number?
Screen Shot
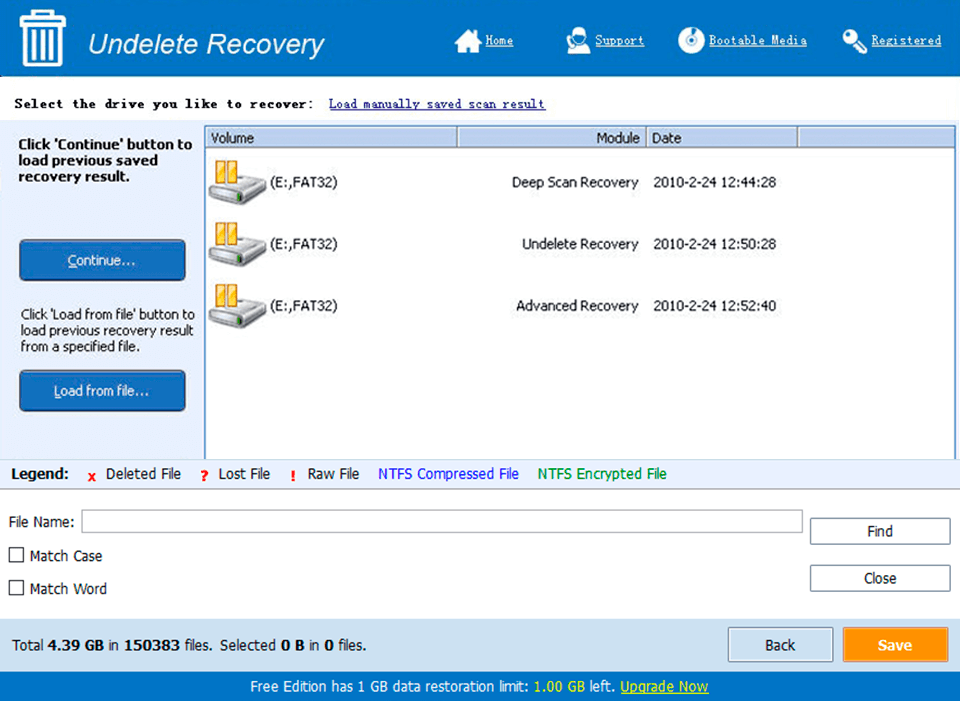
System Requirements for Zip Express 2.7.2.1 serial key or number
- First, download the Zip Express 2.7.2.1 serial key or number
-
You can download its setup from given links:


